Crude oil and natural gas are mixtures of hydrocarbons-chemical molecules that contain only hydrogen and carbon. The simplest hydrocarbon molecule is methane (CH4), it is the essential ingredient of natural gas. Natural gas mixtures divide into two general categories: dry natural gas has high concentrations of methane and ethane (C2H6) (typically > 95%), while rich gases have higher concentrations of propane (C3H8), butane (C4H10), and the intermediate-weight hydrocarbons pentane (C5H12) through heptane (C7H16). This group of hydrocarbons is called alkane or paraffin. As the proportions of heavier molecules in a hydrocarbon mixture increase, it is more likely to exist in liquid form at atmospheric conditions, which is found in condensate and crude oil. In crude oil we can find more complex hydrocarbons such as cycloalkanes (naphthenes) and various aromatic hydrocarbons.


How crude oil and natural gas are formed?
Naturally occurring hydrocarbons come from the decomposed remains of ancient plants and animals. Through a sequence of geologic events that occurred over millions of years, organic material was deposited on the Earth’s surface and then transported to basins, where it accumulated and gradually became buried at great depths under layers and layers of sediments, in what geologists refer to as source rocks and usually are promising targets for oil and gas exploration. Over time, this organic matter subjected to high pressures and temperatures created by the depth, through a series of intermediate chemical reactions, some of this material eventually turned into petroleum. The term petroleum collectively refers to crude oil, natural gas and solid hydrocarbon mixtures like tar and asphalt.
In general, the deeper a rock formation is located in the Earth’s crust, the higher its temperature and pressure will be. Thus, the type of petroleum that formed through these processes depended largely on the depth of the source rocks.
- In relatively shallow source rocks, where temperatures ranged from about 60 to 80°C [140 -176°F], the organic matter was converted into heavy oil.
- At lower depths and higher temperatures, from about 80°C to 175°C [176°F to 347°F], the heavier, long-chain organic molecules began to break up into shorter molecules and form medium and light oil.
- Where temperatures exceeded 175°C [347°F], the molecules became even shorter and lighter, with more and more matter transformed to rich gas until, by the time it had reached 600°F [315°C], all of it had been transformed to dry gas (methane).
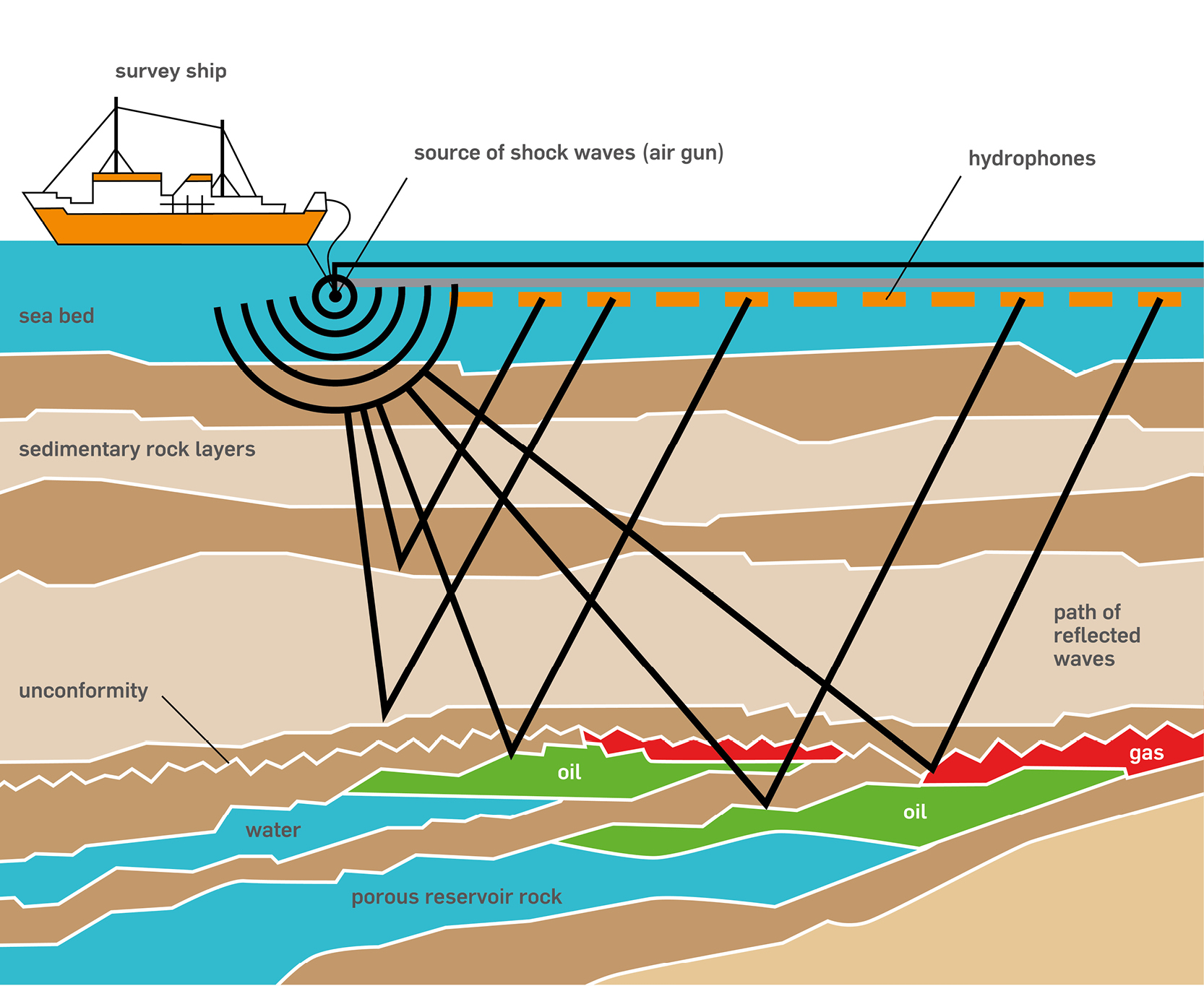
Petroleum Migration and Accumulation
Oil and gas formed in the source rocks are naturally lighter than the water contained in rock formations, moved upward by gravity forces from the source beds along permeable migration paths. Eventually, it accumulated in reservoirs contained within geologic traps, surrounded by impermeable cap rocks or seals that keep it from traveling any farther.
In petroleum industry we often hear the term “reservoir”. An oil and gas reservoir is a porous, permeable rock formation, in which oil and gas are contained in the empty spaces between the rock grains. These spaces are interconnected, thereby forming channels or conduits through which fluids can flow to a well, and from there to the surface.
Formation of reservoir
Separation and Treatment of Produced Oil and Gas at the field
The fluid that comes out of a typical oil well is actually a combination of crude oil and associated gas, often mixed with water, non-hydrocarbon gases (H2S, CO2, N2) and other impurities. That is the reason why the volume of crude oil and natural gas is so different from the reservoir conditions and surface conditions.

Oil Quality:
A crude oil’s density is an important measure of its overall quality. This is because lighter oils are generally easier to refine than heavy oils, and therefore tend to have higher value. Oil density is sometimes expressed in terms of its specific gravity, but more often is given as American Petroleum Institute (API) gravity. API Gravity is a measure of how heavy or light a petroleum liquid is compared to water. If its API gravity is greater than 10, it is lighter and floats on water, if less than 10, it is heavier and sinks.
API gravity = (141,5 / SG) – 131,5
here SG – specific gravity also called relative density, is the ratio of the density of a substance to the density of water at 15,5 °C or 60 °F.

Crude oil and Natural Gas Measurement:
Crude oil is measured in units of volume, weight and thermal energy depend on the purpose of the measurement. For example, petroleum engineers measure oil and gas volumes to answer questions like: How much oil or gas do I have in my reservoir? How much can be produced during the lifecycle of the field? The standard volume unit for crude oil measurement is the 42 gallon barrel (bbl). In countries that use the SI or “metric” system, oil volumes may be measured in metric tonnes.
Natural gas is typically measured in terms of its volume at surface conditions and in thermal energy units. It is measured by weight only when it is in the liquid state (LNG). Because gas is compressible, its volume varies significantly with changes in temperature and pressure. In order for gas volume measurements to have any meaning, they have to have some standard frame of reference. For this reason, the industry has established standard conditions for referring to all gas volumes. In ares like USA, Latin America, Africa and Middle East unit of gas volume measurement in Standard Cubic Foot , whilst Canada, Russia and other countries in Europe, using Standard Cubic Meter.
Crude Oil Refining and Gas Processing:
If the treated gas is dry (contains mostly methane), it may either be compressed and sent directly to its point of sale through high-pressure pipelines, or it may be turned into liquefied natural gas (LNG) by cooling it to temperatures as low as -160ºC [-258ºF]. LNG occupies only about 1/625 of the volume of dry gas, allowing it to be transported to distant markets in specially designed ships.
If the gas is rich (has substantial amounts of ethane or heavier hydrocarbons), one post-treatment option might be to send it to a gas processing plant. There, it may be cooled to around -150ºF [-101ºC], causing these hydrocarbons to condense to natural gas liquids (NGL). The NGL is then sold to a refinery or petrochemical plant. If sufficient NGL is available, it may be economical to separate it further into its constituent hydrocarbons. This involves a process called fractionation in a series of columns, where the NGL is sequentially warmed to higher temperatures, causing the individual hydrocarbons-ethane first, then propane, then butanes-to boil off and then condense. Propane, butanes and their mixtures are referred to as liquefied petroleum gases (LPGs). The residual is Natural Gasoline, which can be stored at atmospheric conditions.

Natural gas, LNG, NGL, LPGs and the hydrocarbons that come from them all have individual markets, and all of them are in their final form to be used as fuels or for manufacturing petrochemicals.
Unlike natural gas crude oils are not suitable for use as finished products, they must be processed at refineries in order to satisfy specific markets.
Overview of refining process
Petroleum products after refining, include gasoline, distillates such as diesel fuel and heating oil, jet fuel, petrochemical feedstocks, waxes, lubricating oils, and asphalt are extremely vital to many other industries.
The refiner’s ultimate goal is to maximize the overall pricing difference between a barrel of crude oil and the petroleum products refined from it, called the crack spread. for example, If the products sell for $60 per barrel and the cost of crude oil is $55 per barrel, then the crack spread is $5 per barrel. We can see that unlike the upstream sector, where profitability of E&P companies is strongly affected by the change of oil prices, the downstream sector is more comfortable with the market change and can persist as long as there is no failure and disruption in operations.